- Home
- Clinical Trial Unit
- Clinical & Research Trials
- Cancer Registry
- Publication
- Useful Links
- Team
- Contact Information
- News & Events
Shifa Clinical Research Center
Director Message
Shifa Clinical Research Center is an affiliate of Shifa International, with a 500 bed JCI accredited Hospital, e-Shifa, Shifa Tameer-e-Millat University, Shifa College of Medicine and Shifa Schools for Nursing & Pharmacology.
SCRC ensures the 4 pillars of any Clinical Trials Unit:
- ROBUST TEAM of Principal and Co-investigators, Clinical Research Associates (CRAs), Physicians, Biostatisticians, Data Managers and Coordinators.
- TRACK RECORD of enrolling thousands of patients in numerous multi-center clinical trials across the globe.
- QUALITY ASSURANCE guided by Joint Commission, Good Clinical Practice principles to ensure ethical, transparent and seamless execution of benchmarks.
- VIABILITY of clinical research as a defining facet of who we are as an organization with leadership committed to advancement of healthcare.
Collaboration is the name of the game in clinical research, and we offer a unique platform in this region unrivalled in bringing the pieces together for meaningful research that changes clinical practice and opens undiscovered avenues.
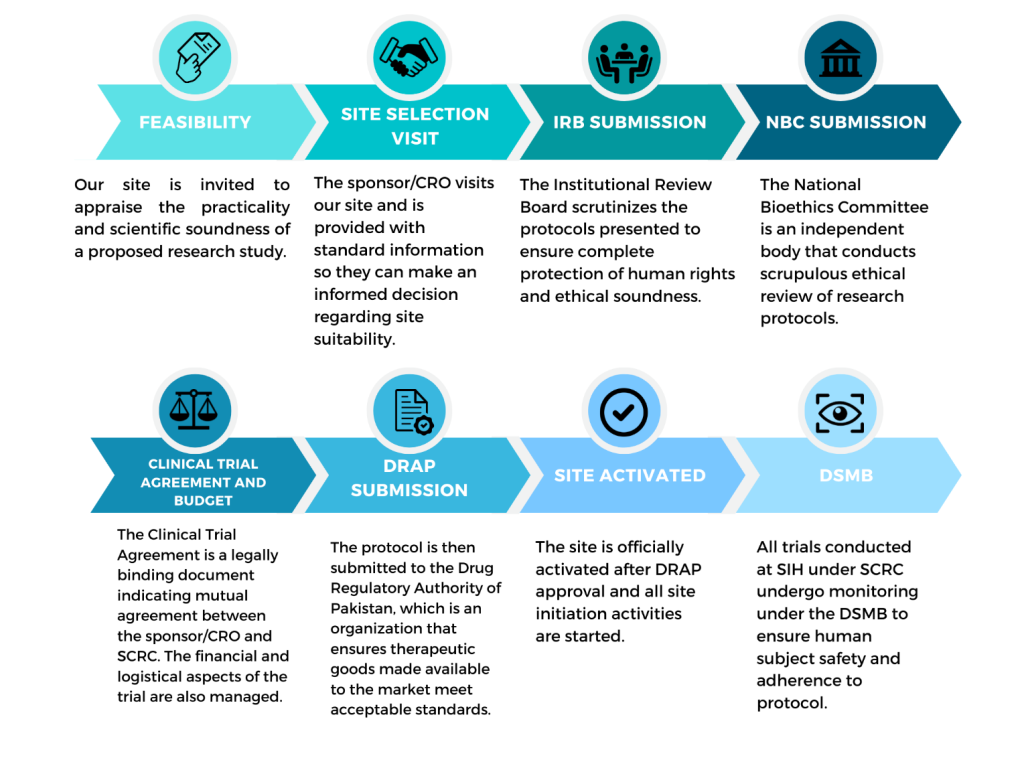
SCRC is a one-stop-shop from study feasibility, identifying PI, forming a team, IRB presentation, Clinical Trial negotiation and agreement, enrollment, follow-up and sorting out AEs until trial is completed.
Our Profile below is a testament to our endeavors.
Dr. Muhammad Ayaz Mir, FACP
Diplomate American Board of Medicine & Hematology
Director, SCRC
pakistanbmt@gmail.com
Shifa Clinical Research Center
Main Office A-0
Inpatient Unit
Research Reception A-0
Research Phlebotomy A-0
Clinical Trial Unit (CTU) A-0
Clinical Trial Repository (CTR) I-9
CTU Clinic
CLINICAL TRIAL APPROVAL PROCESS
Shifa Hospital Research Trials
| TRIALS NAMES | SPONSORS | STATUS |
|
HALT-IT Hemorrhage alleviation with tranexamic acid-intestinal system, Tranexamic acid of the treatment of gastrointestinal bleeding: An International randomized, double-blind placebo-controlled trial. |
 |
Completed Published in Trials https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-019-3561-7 |
|
CRASH-3 Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomized, placebo-controlled trial |
 |
Completed Published in The Lancet https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)32233-0/fulltext |
|
HIP ATTACK 1 Accelerated surgery versus standard care in hip fracture an international, randomized, controlled trial |
Completed Published in The Lancet https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30058-1/fulltext |
|
|
SafeHer Study A phase-III Prospective, two-cohort non-randomized Multicenter, Multinational, open-label study to assess the safety of assisted- and self-administered subcutaneous Trastuzumab as therapy in patients with operable her2-positive early breast cancer |
 |
Completed
https://academic.oup.com/oncolo/article/23/10/1137/6439744
|
|
PERUSE Study A phase III prospective, two-cohort non-randomized, Multicenter, Multinational, open-label single arm study of pertuzumab in combination of as THERAPY in patients with operable Her2-positive early breast cancer |
 |
Completed |
|
POISE-3 Perioperative Ischemic Evaluation-3 |
Completed Published in PubMed, NEJM https://pubmed.ncbi.nlm.nih.gov/35101083/
https://www.nejm.org/doi/full/10.1056/NEJMoa2201171 |
|
|
Can Sino Phase III Trial of A COVID-19 Vaccine of Adenovirus Vector in Adults 18 Years Old and Above |
Completed Published in The Lancet https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02753-7/fulltext |
|
|
COP-AF Colchicine For The Prevention Of Perioperative Atrial Fibrillation In Patients Undergoing Thoracic Surgery |
|
Completed Published in The Lancet, PubMed, European Society of Cardiology
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)01689-6/fulltext
https://pubmed.ncbi.nlm.nih.gov/37640035/
https://www.escardio.org/The-ESC/Press-Office/Press-releases/Colchicine-fails-to-reduce- |
|
Livzon Global Phase 3 Randomized Trial to evaluate efficacy, safety, immunogenicity of Recombinant SARS COV-2 fusion protein vaccine against COVID 19 in healthy adults after vaccination of 2 doses of inactivated vaccines |
 |
Completed Published in Emerging Microbes & Infections https://www.tandfonline.com/doi/full/10.1080/22221751.2022.2088406 |
|
ALVOEYE A Randomized, Double-masked, Parallel-group, MulticenterClinical Study to Evaluate the Efficacy and Safety of AVT06Compared with EU-Eylea® in Subjects with Neovascular (wet)Age-related Macular Degeneration
|
 |
In Progress https://clinicaltrials.gov/ct2/show/NCT05155293
|
|
SEQIRUS A Phase III, Randomized, Observer-blind, Multicenter Study to Evaluate the Efficacy, Immunogenicity and Safety of Seqirus’ Cell-Based Quadrivalent Subunit Influenza Virus Vaccine (QIVc) Compared to a Non-Influenza Vaccine when Administrated in Healthy Subjects aged 6 Months through 47 Months
|
 |
In Progress https://www.clinicaltrials.gov/ct2/show/NCT03165617 |
|
BCD-201 A Randomized, Double-Blind Clinical Study of the Efficacy and Safety of BCD-201(JSC BIOCAD) and Keytruda® in Patients with Unresectable or Metastatic Melanoma |
 |
In Progress |
|
GATES-MRI-301 A Phase 3, randomized, double-blind, placebo-controlled study to evaluate the effect of Bi-26 (strain ofBifidobacterium longum, B. infantis) supplementation versus placebo on weight gain in underweight infants |
 |
In Progress |
|
HIP ATTACK-2 Accelerated surgery versus standard care in hip fracture an international, randomized, controlled trial |
 |
In Progress |
|
ASPIRE-AF Anticoagulation for Stroke Prevention In patients with Recent Episodes of perioperative Atrial Fibrillation after noncardiac surgery |
 |
In Pipeline https://clinicaltrials.gov/ct2/show/NCT03968393
|
|
DEPOSITION Decreasing Postoperative Blood Loss by Topical vs. Intravenous Tranexamic Acid in Open Cardiac Surgery |
 |
In Pipeline https://clinicaltrials.gov/ct2/show/NCT03376061
|
|
BCD-178-2 A Double-Blind, Randomized Clinical Study of the Efficacy and Safety of BCD-178 and Perjeta® as Neoadjuvant Therapy of HER2-Positive Breast Cancer |
 |
In Progress |
Collaborators
The SIH Cancer Registry is a hospital-based registry developed in January 01, 2018. The primary goal of the SIH cancer registry is to collect accurate and complete data on patients diagnosed /treated for cancer in hospital. The non-analytical cases (patients diagnosed on the basis of histopathology from outside Shifa) are not involved in data analysis, but the non-analytical cases were reported as per guidelines of the commission on cancer USA.
Work Flow
Manuals used in Cancer Registry
- The SIH Cancer Registry implemented the WHO, ICD-O3.2 (updated in 2021) coding for topography, morphology, and grade. We used two different staging systems in our cancer registry.
- The American Joint Committee on Cancer (AJCC) and the Surveillance, Epidemiology, and End Results (SEER) 2000. The summary staging is a general staging, and data availability is easy. It applies to the maximum anatomic site, including the brain and lymphomas.
- For treatment purposes, AJCC (TNM) staging is preferred. For multiple primary sites, we utilized MP 2007 SEER Summary manual.
Useful Links
Guidelines for Synopsis and Dissertation Writing for CPSP
National Institute of Health http://www.clinicaltrials.gov
Good Clinical Practices https://gcp.nidatraining.org/
Introduction to the Principles and Practice of Clinical Research
Shifa Clinical Research Center
Block A-0 Shifa International Hospitals Ltd.
Pitras Bukhari Road H-8/4, Islamabad – Pakistan
Telephone:
+92 – 51–8463984
+92 – 51–8463985
+92 – 51–8463385
+92 – 51–8464857
Email: